Context:
Acute Myeloid Leukemias (AML) in children and adolescents represent a group of rare diseases with a high relapse rate (45%) and unfavorable prognosis (<70%) despite therapeutic advances. The ELAM02 therapeutic protocols, the post-ELAM02 registry, and the Myechild01 protocol have enabled the collection of clinical-biological data and the establishment of an AML tumor bank since 2005. The prospective cohort protocol ALARM3 is a research project that began in 2023 and aims to better understand the mechanisms involved in relapse and resistance of pediatric acute myeloid leukemias. Additionally, a multi-center LEA cohort, both historical and prospective, began in 2004. The objective of the LEA program is to describe and understand, through prolonged follow-up, the medium and long-term outcomes of patients treated for a malignant hematopathy during childhood. The retrospective and prospective Co-DOREMy cohort protocol includes children with AML who have not been included in any protocol. The OSCARE protocol, on the other hand, is the secondary AML observatory created in 2019 with retrospective data collection since 2013 and prospective data collection starting in 2021, enabling comprehensive data collection since 2013.
Justification and Purpose of the Study
Due to the rarity of pediatric AML cases, which has only allowed for the description of the most common genetic abnormalities, pooling data will enable a better characterization of rare patient subgroups and establish a treatment response profile. This will facilitate the adaptation of treatment for future patients to reduce relapse rates and improve the prognosis of pediatric AML.
Study Objectives
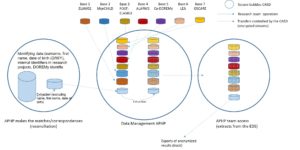
The objectives of the Data Warehouse for Health (DOREMy) project are to integrate all clinical, biological, and anatomopathological data of children with AML into a warehouse to study their various diagnostic, predictive, and prognostic profiles, as well as their interrelations. To achieve this, we propose to establish a Data Warehouse, integrating data from eight research protocols.
Justification of Public Interest
The aim of this project is to study the various diagnostic, predictive, and prognostic profiles, as well as their interrelations (clinical, biological, genetic) of children with AML. Despite numerous research projects on separate cohorts, the prognosis of pediatric AML does not improve.
Our project involves pooling research data and existing clinical-biological data for increased power. The ELAM02, MyeChild01, and post-ELAM02 protocols have included most patients diagnosed with pediatric AML in France. Their detailed clinical and biological data, as well as treatment, toxicities, and outcomes, have been collected in CRFs and monitored. Biological data describing samples stored at Trousseau Hospital or in one of the 6 biology laboratories associated with the project will also be included in this database (as well as any results obtained as part of care or previous research).
As these diseases are rare and the genetic subgroups even rarer, it is crucial to combine all these data sets into a single database to statistically validate our observations.
The ultimate goal of this project is to reduce the relapse rate and improve the survival rate of pediatric AML by identifying rare, uncharacterized patient subgroups that present a high risk of relapse, for which clinical characteristics and outcomes will be compared to omics data, LSC signatures, and drug responses to establish precise and comprehensive profiles.